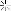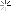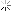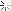I found around 800 sheets of gold foil. I conducted an acid test and the material passed with the 22k solution (the highest one I have). After removing the foil from the paper, I had about 20 grams of gold.
I wanted to turn the foil into a gold bar using an electric melting furnace.
I added gold flakes to the graphite crucible and inserted the crucible into the furnace to heat up.
The temperature reached 1065c. The material was bright red and was smoldering but had not melted. I gradually increased the temperature to 1075c but still no melting.
I emptied the crucible and found a pile of white ash.
Any thoughts on what went wrong?
Thread: Help with melting 24k gold foil
Results 1 to 6 of 6
Threaded View
-
04-01-2020, 11:58 PM #1

- Join Date
- Apr 2020
- Posts
- 2
Help with melting 24k gold foil
Similar Threads
-
Gold Foil laser HELP!
By Kezza in forum Laser Engraving / Cutting Machine General TopicsReplies: 3Last Post: 01-04-2016, 03:42 AM -
Melting gold flakes. . .?
By chroniccodez in forum Casting MetalsReplies: 4Last Post: 05-26-2008, 08:39 PM







 Reply With Quote
Reply With Quote